Breast cancer Intravoxel Incoherent Motion Multisite (BRIMM) Project
Overview
In this multi-site breast cancer study, we use an MRI method called intravoxel incoherent motion (IVIM) to characterize tumor microenvironment and predict response to neoadjuvant chemotherapy.
Advances in the understanding of molecular subtypes of breast cancer have led to improved treatment and outcomes. However, even targeted therapies have mixed results because of the heterogeneity of intratumor microenvironment, underlining how important full characterization of the heterogeneity and complexity of tumors is for the development of effective, personalized imaging biomarkers. Recent research has shown that reducing the variability of quantitative imaging biomarkers can pay tremendous diagnostic and prognostic dividends. However, the development and validation of such biomarkers require crossing institutional, commercial, and analytical boundaries. We address this challenge in the BRIMM project.
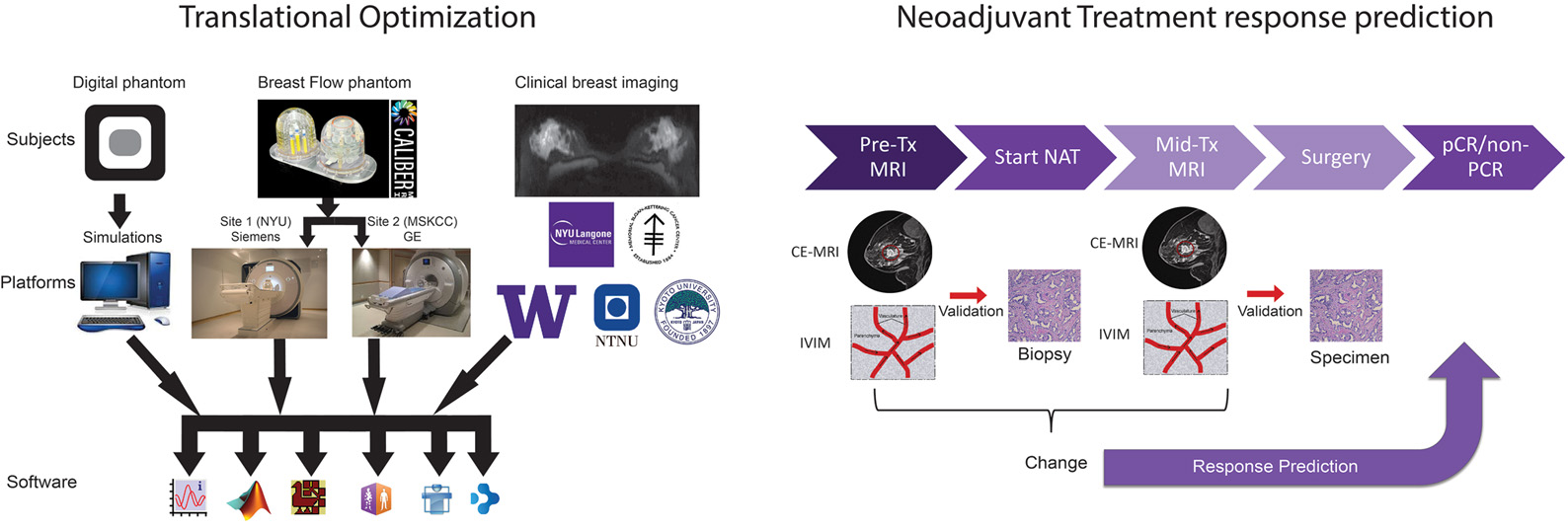
The BRIMM project is a multi-site breast cancer study aimed at developing a robust, translation-ready technique for predicting response to neoadjuvant chemotherapy by targeting the chemotherapy setting with a quantitative MRI method called intravoxel incoherent motion (IVIM). This approach probes two key features of the tumor microenvironment—angiogenesis and cellularity—noninvasively. A successful method has the potential to allow non-responding breast cancer patients to change treatment, leading to lower toxicity and better response.
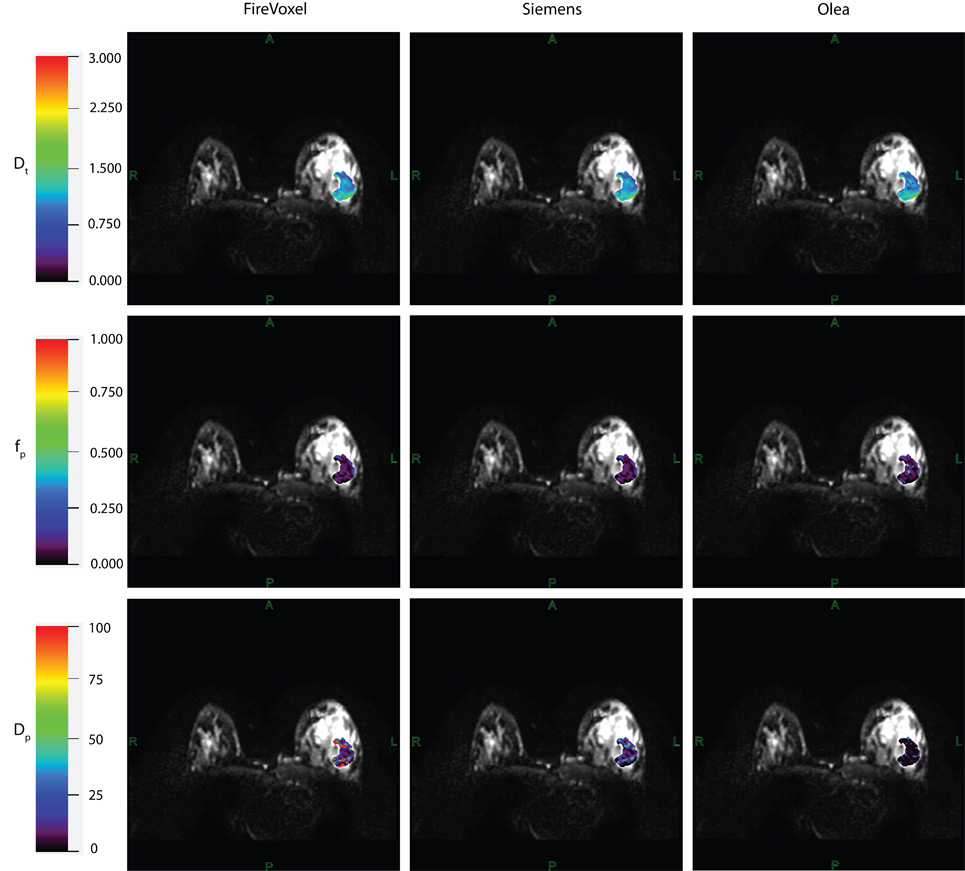
The project proceeds in two phases. First, we assess IVIM measurement variability across five collaborating sites and maximize cross-platform accuracy for clinical implementation of IVIM in breast cancer patients. This includes the use of retrospective clinical data, digital-phantom data and a manufacturable breast flow phantoms. Second, we conduct prospective studies at two sites, focusing on the IVIM parameters shown to be robust predictors of response. In this phase, we collect IVIM from enrolled patients prior to and early in their treatment, and analyze it in combination with all other acquired data to generate a best practices algorithm for application in future breast cancer neoadjuvant therapy trials.
Keywords
- Intravoxel Incoherent Motion
- Diffusion MRI
- Breast Cancer
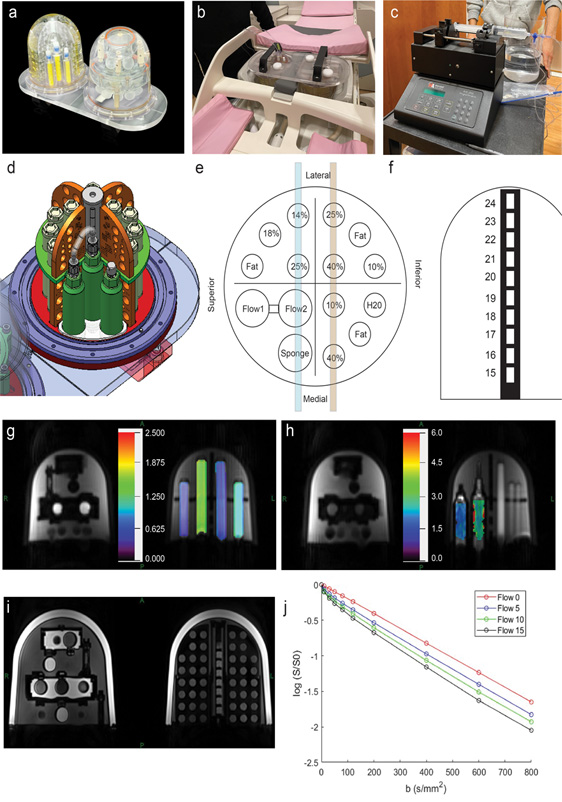
Figure 1. (a-e): Flow phantom, syringe pump, and DWI slices in (g,h). (f) Transition temperatures of liquid crystal (LC) elements. (g, h): ADC maps in PVP vials (g) and sponge compartments (h), showing elevated ADC with flow. (i) T1-weighted MRI of LC elements. (j) IVIM signal decays vs. flow rate in mL/min.
Project Team

Eric E. Sigmund, PhD
Project Lead
External Collaborators
- Sunitha Thakur, PhD (Project Lead) , Memorial Sloan Kettering Cancer Center
- Judith D. Goldberg, ScD, NYU Langone Health
- Xiaochun Li, NYU Langone Health
- Farbod Darvishian, MD, NYU Langone Health
- Nancy Chan, MD , NYU Langone Health
- Varadan Sevilimedu, DrPH , Memorial Sloan Kettering Cancer Center
- Sarah Eskreis-Winkler, MD, PhD , Memorial Sloan Kettering Cancer Center
- Dilip D. Giri, MD, FACP , Memorial Sloan Kettering Cancer Center
- Komal Jhaveri, MD, FACP , Memorial Sloan Kettering Cancer Center
- Katja Pinker-Domenig, MD, PHD , Columbia University Irving Medical Center
- Savannah Partridge, PhD , University of Washington
- Debosmita Biswas , University of Washington
- Tone Bathen , Norwegian University of Science and Technology
- Mami Iima , Nagoya University
- Maya Honda , University of Kyoto
- Masako Kataoka , University of Kyotoy
- Oliver Gurney-Champion , Amsterdam UMC
Publications
- Basukala Basukala D, Mikheev A, Li X, et al. Retrospective BReast Intravoxel Incoherent Motion Multisite (BRIMM) multisoftware study. Front Oncol. 2025;15:1524634. Published 2025 Feb 24. doi:10.3389/fonc.2025.1524634
- Basukala D, Mikheev A, Sevilimedu V, et al. Multisite MRI Intravoxel Incoherent Motion Repeatability and Reproducibility across 3 T Scanners in a Breast Diffusion Phantom: A BReast Intravoxel Incoherent Motion Multisite (BRIMM) Study. J Magn Reson Imaging. 2024;59(6):2226-2237. doi:10.1002/jmri.29008
- Honda M, Sigmund EE, Le Bihan D, et al. Advanced breast diffusion-weighted imaging: what are the next steps? A proposal from the EUSOBI International Breast Diffusion-weighted Imaging working group. Eur Radiol. 2025;35(4):2130-2140. doi:10.1007/s00330-024-11010-0
Acknowledgements
We acknowledge support from the following NIH grants: UG3CA239861, UH3CA239861, P41 EB017183, P30CA016087, P30CA008748, R01CA207290, R01CA248192, R01CA190299.