Neuroimaging Core of NYU Langone’s Alzheimer’s Disease Research Center

Overview
The neuroimaging core of NYU Langone’s Alzheimer Disease Research Center (ADRC) provides imaging expertise to support ADRC’s mission of advancing research on Alzheimer’s disease and related dementias (ADRD) and developing novel diagnostics and treatments.
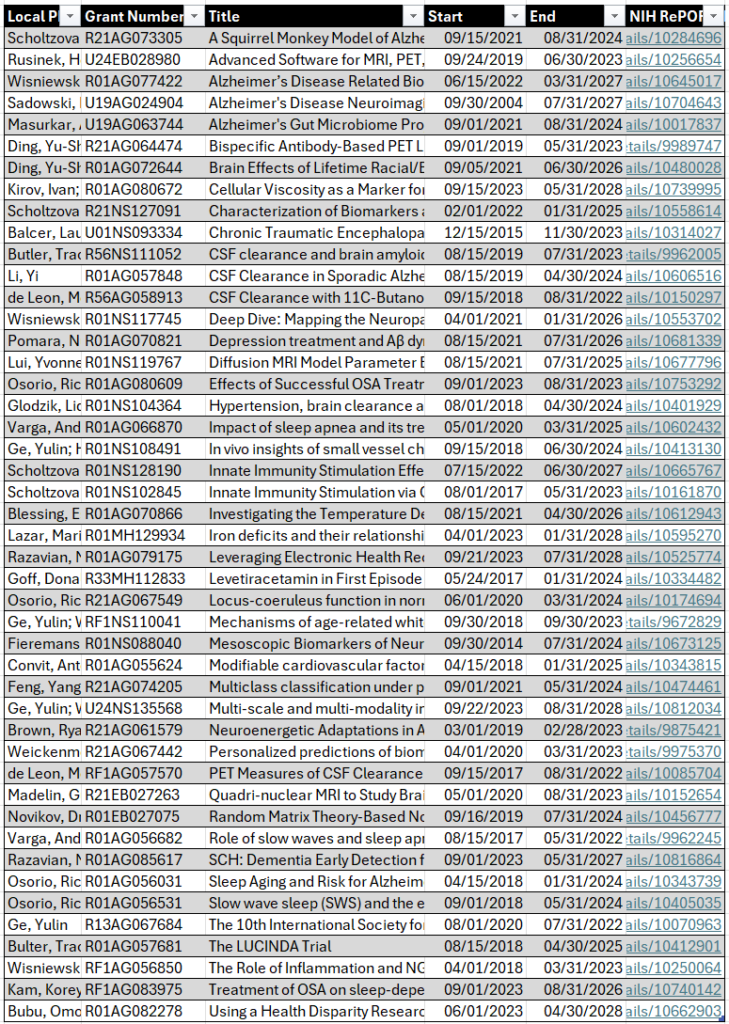
Established in 1990, NYU Langone’s ADRC has performed transformative research on ADRD, including on disease heterogeneity, vascular risk factors, and the timing of the transition from normal aging to subjective cognitive decline, mild cognitive impairment, and early dementia. The center follows a cohort of 400 study participants who range from cognitively normal to diagnosed with early dementia. The participants are phenotyped and assessed using neuropsychological testing, imaging, and state-of-the-art plasma and cerebrospinal fluid biomarker assays.
The neuroimaging core provides imaging protocols, develops and validates new imaging modalities and analysis methods, and supports ADRC’s investigators—all in alignment with the NIH Alzheimer’s Project Plan.
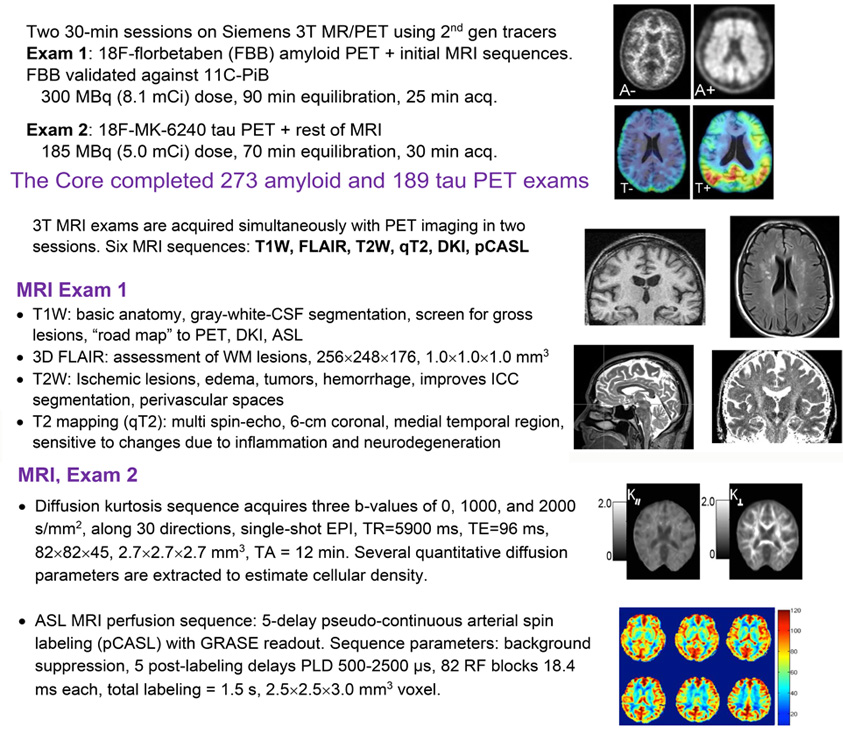
The core maintains a longitudinal PET/MR protocol that combines PET imaging of amyloid and tau accumulation with MR sequences for measuring cortical thickness, white matter lesions, T2 relaxation, perfusion, and diffusion. Images are shared with national repositories and initiatives, such as SCAN, NACC, CLARiTI and DVCID, contributing to broadly available imaging resources for further research. In collaboration with NYU Langone’s ADRC neuropathology core, the neuroimaging core also correlates in vivo and postmortem MRI with histology-based interpretation of imaging features.
To-date, the work of the neuroimaging core has significantly contributed to the early diagnosis of Azheimer’s through linking changes on structural MRI with histological data and developing dynamic PET models of the flow of cerebrospinal fluid. In addition, the advanced structural and functional brain imaging metrics developed by NYU Langone’s ADRC imaging core are being applied in affiliated projects to investigate the impact of sleep disturbances, hypertension, and obesity on accelerated cognitive decline and Alzheimer’s progression.
Keywords
- Brain Aging
- Cognitive Decline
- Dementia
- Protocol Standardization
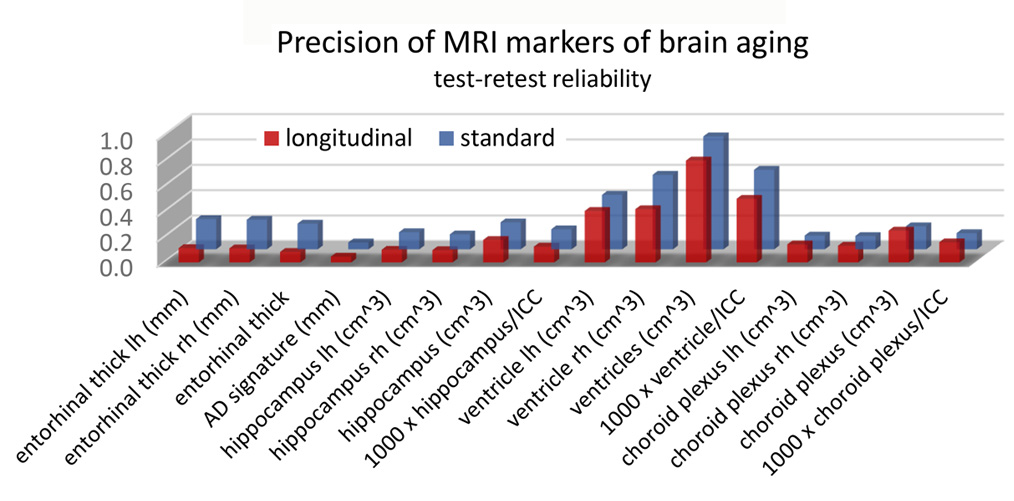
Figure 2. One of the functions of NYU Neuroimaging core located at CBI is to validate potential biomarkers of neurodegeneration. As part of this effort, we must estimate the repeatability of structural brain measures. Here we compare results from two T1-weighted 3T MRIs, acquired with a short time interval, in 100 consecutive NYU ADRC participants. FreeSurfer 7.1 segmentation software was applied in both standard and longitudinal mode. The demographics were: age, 73.5±6.1 years, 64% women, 9-20 years education, time interval between exams 5.5±5.2 weeks. Plotted is the standard deviation of the difference between 16 often-used biomarkers. It is seen that the longitudinal mode improves the test-retest reliability for most measures, especially for the entorhinal cortex thickness.
Project Team

Henry Rusinek, PhD
Project Lead

Timothy M. Shepherd, MD, PhD
Project Lead
External Collaborators
- Thomas M. Wisniewski, MD , NYU Langone Health
- Ricardo M. Osorio Suarez, MD, NYU Langone Health
- Arjun V. Masurkar, MD, PhD, NYU Langone Health
- Omonigho M. Bubu, MD, MPH, PhD, NYU Langone Health
- Nobuyuki Okamura, Japan
- Johannes Weickenmeier, University of Oxford
- and many others...
Publications
- Tian Y, Rusinek H, Masurkar AV, Feng Y. ℓ1-Penalized Multinomial Regression: Estimation, Inference, and Prediction, With an Application to Risk Factor Identification for Different Dementia Subtypes. Stat Med. 2024;43(30):5711-5747. doi:10.1002/sim.10263
- Mao A, Flassbeck S, Marchetto E, Masurkar AV, Rusinek H, Assländer J. Sensitivity of unconstrained quantitative magnetization transfer MRI to Amyloid burden in preclinical Alzheimer’s disease. Preprint. medRxiv. 2024;2024.04.15.24305860. Published 2024 Jul 7. doi:10.1101/2024.04.15.24305860
- Flaherty R, Sui YV, Masurkar AV, Betensky RA, Rusinek H, Lazar M. Diffusion imaging markers of accelerated aging of the lower cingulum in subjective cognitive decline. Front Neurol. 2024;15:1360273. Published 2024 May 9. doi:10.3389/fneur.2024.1360273
- Caçoilo A, Dortdivanlioglu B, Rusinek H, Weickenmeier J. A multiphysics model to predict periventricular white matter hyperintensity growth during healthy brain aging. Brain Multiphys. 2023;5:100072. doi:10.1016/j.brain.2023.100072
- Li C, Rusinek H, Chen J, et al. Reduced white matter venous density on MRI is associated with neurodegeneration and cognitive impairment in the elderly. Front Aging Neurosci. 2022;14:972282. Published 2022 Sep 1. doi:10.3389/fnagi.2022.972282
- Liu S, Masurkar AV, Rusinek H, et al. Generalizable deep learning model for early Alzheimer’s disease detection from structural MRIs [published correction appears in Sci Rep. 2023 Oct 2;13(1):16528. doi: 10.1038/s41598-023-43726-2.]. Sci Rep. 2022;12(1):17106. Published 2022 Oct 17. doi:10.1038/s41598-022-20674-x
Acknowledgements
This work was supported by the NIH grant P30 AG066512 from NIAs and U24 NS135568 from NIND.